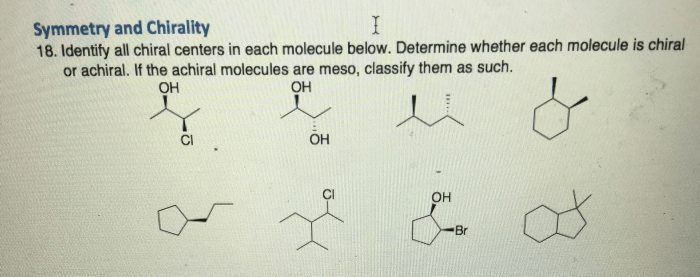
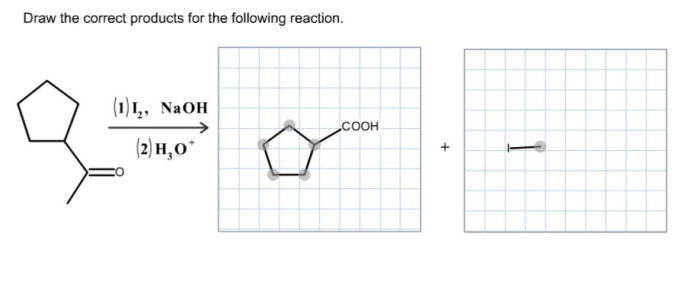
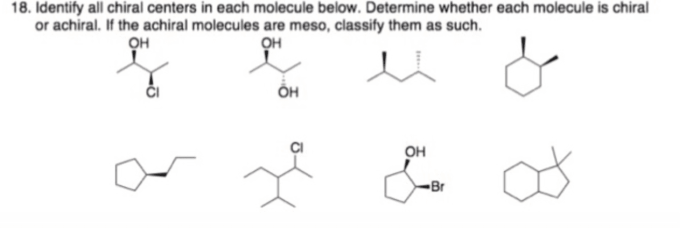
Identify each of the following structures as chiral or achiral. – Chirality and achirality are fundamental concepts in chemistry, describing the symmetry and handedness of molecules. Identifying chiral and achiral structures is crucial for understanding their properties and applications. This guide provides a comprehensive overview of chirality, methods for its identification, and its significance in various fields.
Chirality and Achirality
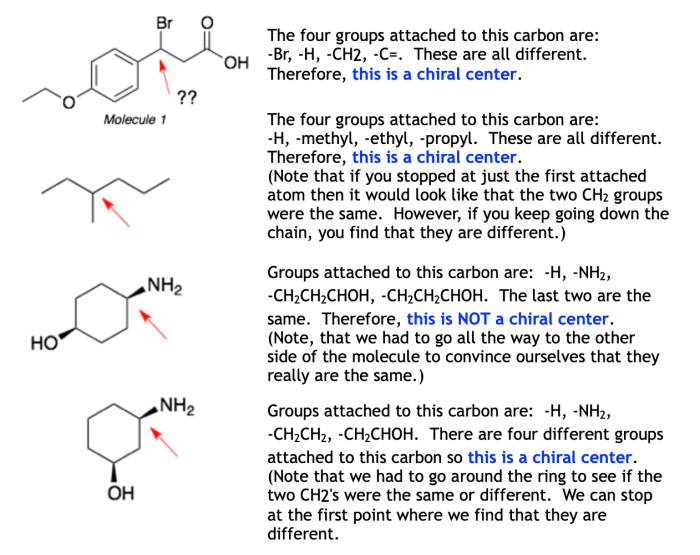
Chirality refers to the property of an object that is not superimposable on its mirror image. In other words, a chiral object has a handedness, like a right hand and a left hand. Achirality, on the other hand, refers to the property of an object that is superimposable on its mirror image.
Identifying chiral and achiral structures is significant because it has implications in various fields, including drug design, the food industry, and materials science. For instance, in drug design, the chirality of a drug molecule can affect its efficacy and toxicity.
Similarly, in the food industry, the chirality of food additives can affect their flavor and aroma.
Methods for Identifying Chirality, Identify each of the following structures as chiral or achiral.
There are several methods for identifying chiral and achiral structures. One common method is the mirror image test. If an object is not superimposable on its mirror image, then it is chiral. Another method is to examine the molecular symmetry of an object.
If an object has a plane of symmetry, then it is achiral. Finally, optical activity can also be used to identify chiral structures. Chiral molecules exhibit optical activity, which means they rotate plane-polarized light.
Examples of Chiral and Achiral Structures
There are numerous examples of chiral and achiral structures. Some examples of chiral molecules include lactic acid and limonene. Lactic acid is a chiral molecule that exists in two enantiomeric forms, which are mirror images of each other. Limonene is a chiral molecule that is found in citrus fruits.
It also exists in two enantiomeric forms, which have different odors.
Some examples of achiral molecules include methane and carbon dioxide. Methane is an achiral molecule because it has a tetrahedral shape and is superimposable on its mirror image. Carbon dioxide is an achiral molecule because it has a linear shape and is also superimposable on its mirror image.
Applications of Chirality
Chirality has a wide range of applications in various fields. In drug design, the chirality of a drug molecule can affect its efficacy and toxicity. For example, the enantiomers of the drug thalidomide have different effects on the body. One enantiomer is a sedative, while the other enantiomer is a teratogen, which can cause birth defects.
In the food industry, the chirality of food additives can affect their flavor and aroma. For example, the enantiomers of the amino acid aspartame have different tastes. One enantiomer is sweet, while the other enantiomer is bitter.
In materials science, chirality can be used to create materials with unique properties. For example, chiral materials can be used to create optical filters and sensors.
Q&A: Identify Each Of The Following Structures As Chiral Or Achiral.
What is the difference between chirality and achirality?
Chirality refers to molecules that are not superimposable on their mirror images, while achirality refers to molecules that are superimposable on their mirror images.
How can I determine if a molecule is chiral?
You can use the mirror image test, examine molecular symmetry, or measure optical activity to determine chirality.
Why is chirality important in drug design?
Chirality can affect the biological activity, metabolism, and toxicity of drugs, making it crucial for designing effective and safe pharmaceuticals.